Folic Acid Risk Assessment
On this page
Skip the menu of subheadings on this page.Risk Assessment:
What is the risk of allergic reactions to folic acid in UK consumers if non-wholemeal flour is fortified with folic acid at 250 µg per 100 g without its presence being labelled on the packaging of the final food or, in the case of food sold loose, not conveyed by other means during a 3 month derogation period?
Risk Assessment Unit
Science, Evidence and Research Division, FSA
Version 6.0
02 October 2023
1. Executive Summary
This risk assessment considers the risk in terms of hypersensitivity to UK consumers if folic acid is used to fortify non-wholewheat flour at 250 µg per 100 g without its presence being labelled on the packaging or not conveyed by other means during a 3 month derogation period.
The UK prevalence of hypersensitivity to folic acid is not known. Leading UK allergy specialists and the UK wide charity operating for people at risk from severe allergic reactions and anaphylaxis were contacted to inform the risk assessment and were not aware of evidence of hypersensitivity to folic acid in the UK. A small number of cases have been reported in the literature although these were linked to supplements rather than the consumption of food.
An allergen reference dose for folic acid has not been established and so the usual approach for assessing hypersensitivity risk could not be followed. Instead, the 75th and 97.5th percentile amount of folic acid that would be consumed if non-wholemeal flour is fortified at the proposed level was estimated and found to be lower than the amount reported to have caused adverse reactions from supplements described in the published literature, with the exception of two cases.
This suggests that while it may be possible for the proposed amount of folic acid in fortified non-wholemeal wheat flour to trigger reactions, this is only likely to occur very rarely in highly sensitive individuals and is not significant on a population basis.
Symptoms of the reported adverse reactions to folic acid supplements range from mild to severe (including anaphylaxis) although no deaths have been reported in the literature. There are currently no reports of hypersensitivity to folic acid in food.
Overall, if non-wholemeal flour is fortified with folic acid at 250 µg per 100 g without its presence being labelled on the packaging of the final food or, in the case of food sold loose, not conveyed by other means during a 3-month derogation period, then we estimate the risk of hypersensitivity to folic acid in UK consumers to be as follows:
- The frequency of adverse reactions to folic acid in food to be very low (i.e., very rare but cannot be excluded).
- The severity of illness in relation to adverse reactions to folic acid in food to be medium (i.e., moderate illness: not usually life-threatening, sequelae rare, moderate duration).
- The level of uncertainty to be medium (i.e., there are some but no complete data available; evidence is provided in small number of references).
2. Statement of Purpose
This risk assessment focuses on the immediate acute hazard of concern in terms of adverse reactions in UK consumers if folic acid is used to fortify non-wholemeal wheat flour. This risk assessment is intended to apply to an approximate three-month transitional period, whereby fortified non-wholewheat flour containing products will not have the presence of folic acid declared on labels.
It should be noted that this risk assessment does not cover consumption of final products made outside the UK or dietary intake from other sources of folic acid or folate, including dark green leafy vegetables, beans, fresh fruit, aquatic foods, eggs and other foods fortified with folic acid such as cereals, spreads and supplements where this is already labelled.
This risk assessment uses the term hypersensitivity when referring to adverse reactions to folic acid because folic acid is not considered a food allergen and the mechanisms of adverse reactions to folic acid are unknown. Food hypersensitivity is a general term which includes food allergies, intolerances and coeliac disease. Allergy is a specific type of hypersensitivity reaction, which involves the immune system (Tanno et al, 2016). In food, this reaction is typically mediated by IgE antibodies, which detect allergenic proteins and present them to other cells in the immune system (Food Standards Agency, 2016).
3. Background
The neural tube is part of the developing foetus’s nervous system, and its development can be impaired for a number of reasons including folate deficiency during early pregnancy. The number of neural tube affected pregnancies (e.g. spina bifida and anencephaly) in the UK remains a concern to UK Health Departments. Advice to women to take folic acid supplements prior to conception and up to the 12th week of pregnancy has been in place for many years but this public health issue remains, particularly for unplanned pregnancies where a woman may not find she is pregnant until well into the crucial 12-week period when the neural tube is closing.
UK Health Departments plan to implement a public health intervention (Consultation – ‘Amending the Bread and Flour Regulations 1998 and the Bread and Flour Regulations (Northern Ireland) 1998’). The proposed intervention requires an amendment to the Bread and Flour Regulations lead by the Department of Health and Social Care (DHSC) and Department for Environment, Food and Rural Affairs (Defra), and requires food business operators to fortify flour (at the milling stage) with folic acid at a level of 250 µg per 100 g of non-wholemeal wheat flour. This level was chosen based on the impact model commissioned by Food Standards Scotland (FSS) (Stochastic modelling to estimate the potential impact of fortification of flour with folic acid in the UK).
The fortified flour will be used in a wide range of food products, which in turn will increase folic acid consumption across the UK, including by pregnant women, thereby reducing the likelihood of neural tube defect-affected pregnancies.
Food Business Operators using the flour products will need to ensure that labelling reflects the addition of folic acid when flour mills start adding it to the flour. However, concerns have been raised that there will be challenges in changing labelling on such a vast array of products and so it has been suggested that there could be transitional arrangements to facilitate the change, which could mean that for a 3-month period within an overall period of up to 2 years (a) folic acid is not present in products labelled as containing it or (b) products contain folic acid but this is not reflected on the label.
FSA has therefore been asked to consider whether this could present a food allergy risk, for example because the risk of anaphylaxis from folic acid has been highlighted by NHS advice (Side effects of folic acid – NHS (www.nhs.uk), although it is unclear whether these concerns are relevant to food hypersensitivity as opposed to reactions to supplements or medicines containing folic acid.
The Committee on the Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) previously assessed the safety of folic acid (Folic Acid Statement Final (food.gov.uk); whilst this did not focus on allergenicity the Committee did comment that “a small number of case reports have described hypersensitivity reactions to oral folic acid therapy (generally more than/equal to 1 mg/day). One short-term, uncontrolled supplementation trial reported adverse symptoms (mental changes, sleep disturbances and gastrointestinal symptoms) in healthy volunteers given very high doses of folic acid (15 mg/day) for one month, but other studies have not observed similar effects”.
4. Hazard Identification
In the UK there are 14 types of food or food groups that are recognised as allergenic foods of public health importance and therefore regulated. These are celery, cereals containing gluten, crustaceans, eggs, fish, lupin, milk, molluscs, mustard, nuts, peanuts, sesame seeds, soya and sulphur dioxide. Folic acid is not included in the list of 14 regulated allergens. However, adverse reactions to folic acid supplements have been reported in the literature.
Folic acid hypersensitivity
As opposed to most food allergens, folic acid is not a protein but a synthetic form of vitamin B9 which is fully oxidised (Gaeta, et al. 2020). Folate is a general term used to describe various forms of vitamin B9 including folic acid, dihydrofolate (DHF), tetrahydrofolate (THF), 5,10-methylenetetrahydrofolate (5,10-MTHF), and 5-methyltetrahydrofolate (5-MTHF) (Centre for Disease Control and Prevention, 2022).
Since folic acid is a synthetic form of folate, it is not found naturally in foods (Gaeta, et al. 2020) and therefore it is important to clarify that the terms cannot be used interchangeably. Hypersensitivity reactions have been reported only for this synthetic form of folic acid (in supplements) and not for naturally occurring folates in food.
Folic acid in its synthetic form has higher bioavailability than natural folate, meaning more can be taken into the body and utilised in comparison to natural folate found in foods. Folic acid can be detected in the blood at doses as low as 200 µg once saturation levels in the red blood cells are reached (Schrijvers et al. 2015).
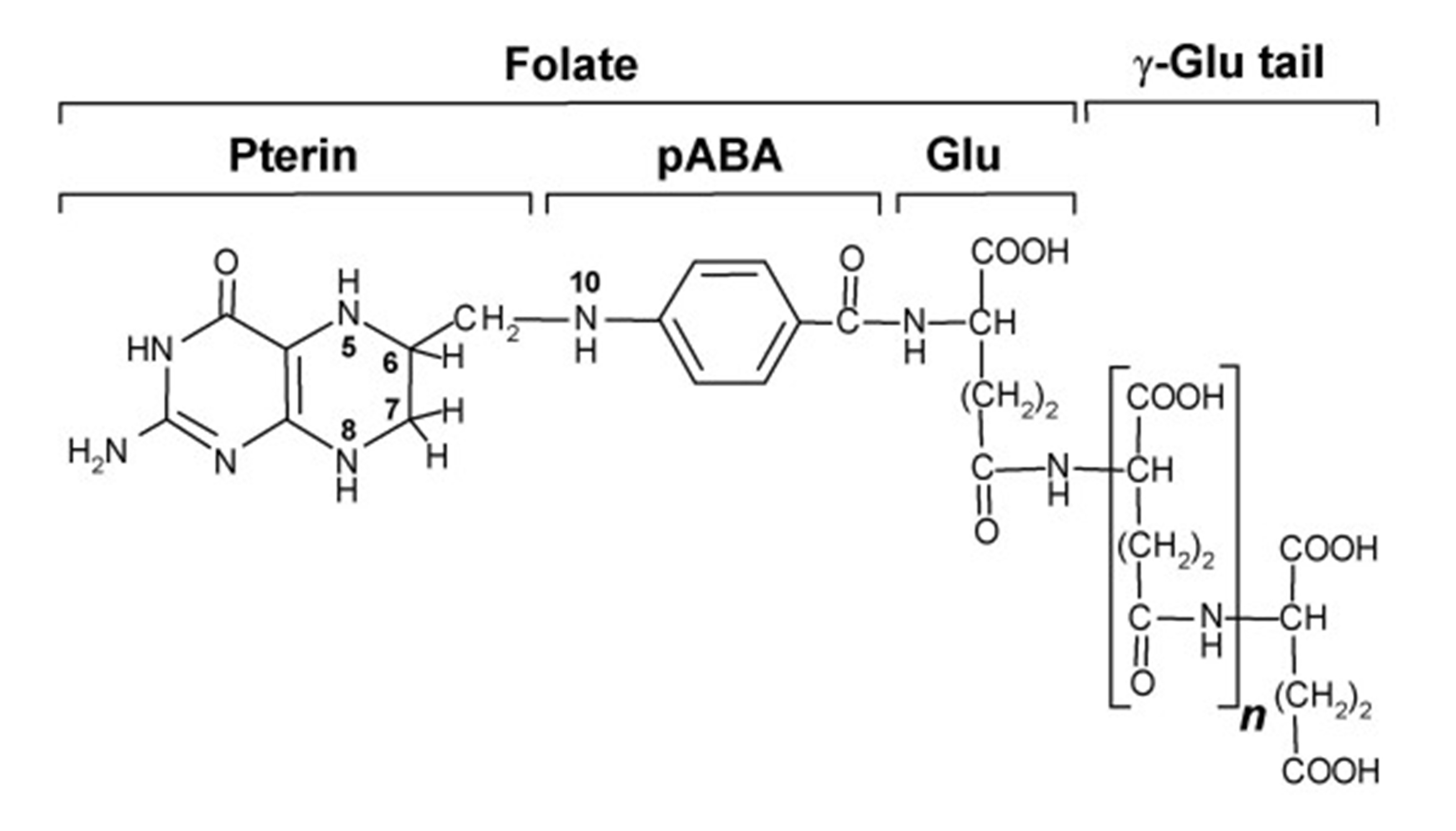
Dietary folates consist mainly of polyglutamate forms of tetrahydrofolate (THF) as seen in Figure 1 (Ebara, 2017). THF is hydrolysed in the small intestine by an enzyme (glutamate carboxypeptidase II) into the monoglutamate form, where it enters the natural folate metabolism cycle and undergoes a two-step process converting it firstly to 5,10-methylenetetrahydrofolate (5,10-MTHF), and finally to 5-methyltetrahydrofolate (5-MTHF), in which form it is transported in the bloodstream to the body cells and tissues (Ebara, 2017, Apraxine et al. 2022).
Figure 1. The structure of natural folate found in food, tetrahydrofolate (THF). Source: Crécy-Lagard et al. (2007).
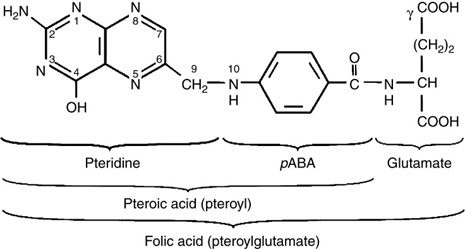
This process differs from that for synthetic folic acid, which is already in a monoglutamate form (Figure 2), therefore does not need to undergo hydrolysis. One key difference to natural folates is that synthetic folic acid must undergo reduction and methylation prior to entering the natural folate metabolism cycle. This process is carried out by an enzyme (dihydrofolate reductase), which converts folic acid to THF and this takes place in the liver (Milman, 2012). Once the reduction of folic acid is complete, it follows the same pathway as that of natural folates, being converted to 5,10-MTHF and finally 5-MTHF.
Figure 2. The structure of monoglutamate synthetic folic acid. Source: Andlid et al. (2017).
Although there is knowledge of the pathways of metabolism for both folic acid and folates alongside clear knowledge of how the structures differ, there is no information in the literature explaining how or why synthetic folic acid may cause hypersensitivity reactions.
5. Hazard Characterisation
Uses of folic acid
Folic acid is widely used as a fortificant in foods such as breads and breakfast cereals, but this is currently on a voluntary basis. The Expert Group on Vitamins and Minerals (EVM) reviewed the occurrence of folic acid in foods in 2002 and estimated that 80-90% of breakfast cereals consumed have been fortified with folic acid with the exception of muesli, which is not normally fortified. It was stated that most products that have been fortified with folic acid contain between 125 and 200 µg/100g, although some products can contain substantially higher amounts (333 µg/100g). Fortification of bread is less widespread and mainly found in soft grain varieties, with approximately 120 µg/100g (Department of Health, 2000). Some low-fat spreads are also fortified with up to 200 µg/20g. These values are based on data obtained for Great Britain (EVM, 2002). In the UK there have been more calls for the mandatory fortification of some food products with folic acid (EVM, 2002).
Folic acid is also used as a dietary supplement and women of child-bearing age trying to conceive are advised to take 400 µg/day prior to conception and for the first 12 weeks of pregnancy (Scientific Committee on Food (SCF), 2000; NHS, 2022). Folic acid can also be used for the treatment of folate deficiency anaemia or if a patient has been prescribed methotrexate (an immunosuppressant used in the treatment of certain inflammatory conditions such as psoriasis, Crohn’s disease and rheumatoid arthritis, and also of some types of cancers).
Other countries have already started using folic acid as a mandatory fortificant in food. In 1998, the U.S Food and Drug Administration (FDA) required 140 µg folic acid / 100g to be added to enriched breads, cereals, flours, cornmeal’s, pastas, rice and other grain products to reduce the risk of neural tube defects. The fortification programme increased mean folic acid intake in the US in 2002 by about 190 µg/day. In 2016, the FDA approved voluntary addition of up to 154 µg folic acid/100g to masa (corn) flour (National Institute of Health (NIH), 2022). Canada have also required folic acid to be added to many grains including white flour, cornmeal and enriched pasta at 150 µg folic acid / 100g since 1998. There are now around 80 other countries that have established folic acid fortification programmes, including Costa Rica (180 µg/100g), Chile (220 µg/100 g flour) and South Africa (150 µg/100 g), which has been associated with a decrease of 19-55% in NTD cases (CDC, 2010; Wald, 2022).
Prevalence and severity of folic acid hypersensitivity
The worldwide literature on folic acid hypersensitivity is limited and true prevalence is unknown in the UK. There are no adverse reactions reported in the literature associated with consumption of foods fortified with folic acid. The following studies from around the world show examples of adverse reactions to folic acid supplements. Some of the cases below also reported positive skin prick or intradermal tests with folic acid, but it is important to note that there are no validated tests for folic acid sensitivity available on the market.
The earliest case report was from 1949, which reported a patient who developed maculopapular dermatitis during a course of oral folic acid treatment at 15 mg/day for 2 weeks. Subsequently, the patient suffered a severe anaphylactoid reaction following intravenous administration of 50 mg of folic acid (Mitchell et al. 1949). However, there is no information on the nature of the symptoms or whether the patient consumed other products to which they could have been sensitised.
Chanarin et al. (1957) reported a case of a male volunteer who, after taking 20 mg folic acid in tablet form, developed symptoms of general malaise, aching pain in the lower thoracic region, itching and general pruritus and breathing difficulties. The patient had previously been given 3 mg of oral folic acid as a solution with no adverse effects.
A report published in 1966 described the case of a 9-month-old infant who displayed adverse reactions (including urticaria) on two separate occasions after treatment with 5 mg folic acid tablets (Mathur, 1966). This was followed by a positive intradermal test for folic acid sensitivity. Woodliff and Davis (1966) also described an adverse reaction in two patients after intravenous administration of folic acid.
A 36-year-old male experienced pruritus on beginning 1 mg/day oral folic acid supplementation, with the symptoms disappearing once treatment stopped but recurring when treatment was restarted three months later (Sesin and Kirschenbaum, 1979).
Sparling and Abela (1985) reported a case of a 62-year-old male who suffered a severe hypersensitivity reaction (bronchospasm, generalised itchy rash) after taking one 5 mg folic acid tablet and suffered a similar reaction to 5 mg folic acid given in a sugar base. This was followed by a positive intradermal test for folic acid sensitivity.
In 2000, Dykewicz et al. reported on a 32-year-old woman who developed urticaria, facial angioedema, nausea and repeated vomiting 20 minutes after ingesting a multivitamin tablet. After receiving treatment at hospital and recovering it was noted that she had had a similar reaction to a different brand of multivitamin years earlier, but not to a liquid high-potency vitamin mixture. The constituents present in the multivitamin but absent from the liquid formulation were folic acid and yellow dye No. 6. A skin prick test was positive for folic acid sensitivity but negative for yellow dye No. 6 sensitivity.
Another case that was reported in 2007 was of a woman who had adverse reactions, including anaphylaxis, after taking synthetic folic acid. The first episode was after taking a 5 mg folic acid tablet where she suffered from an itchy throat, nausea, generalised rash, diarrhoea and light headedness, which was treated by antihistamines. The second episode occurred after she consumed 800 mL of lime flavoured water that was fortified with 20 µg/100 mL folic acid. Again, she suffered from an itchy throat, nausea and generalised pruritus and was treated with adrenaline and antihistamines. The final episode occurred after she consumed 150 mL of a beverage containing feijoa (a fruit of the Myrtaceae family) and supplements including 53.5 µg/100 mL folic acid. She was administered adrenaline en route to hospital after suffering generalised rash, vomiting and light-headedness. Intradermal testing for folic acid sensitivity was positive (Smith et al. 2007).
Schrijvers et al. 2015 reported a case of a 53-year-old woman who presented with anaphylactic shock 10 minutes after consuming a 5 mg folic acid tablet, along with prednisone, vitamin B1 and B6 complexes, magnesium oxide and simethicone. She received treatment with adrenaline, antihistamines, corticosteroids and volume expanders. She reported tolerating the same dose of folic acid the previous day but had milder symptoms of pruritus, flush, diarrhoea after consuming beverages and food fortified with vitamins which included folic acid. The skin prick test was positive for folic acid sensitivity. The patient was advised to avoid foods and beverages fortified with folic acid and given an allergy card and rescue medication.
In 2018, Nucera et al. reported 3 new case studies of hypersensitivity to folic acid. The first 2 cases (one male, one female) were both experiencing severe anaemia and were treated with 5 mg of folic acid. The female suffered with generalised urticaria and was treated with oral antihistamines. It was noted that she had a long history of allergic rhinoconjunctivitis (grass pollen and wall pellitory). The male lost consciousness within 15 minutes of administration of the folic acid and was treated with intravenous steroids and antihistamines; he had no previous allergies reported. Both patients had positive skin prick tests with folic acid and both patients were diagnosed with an allergy to folic acid.
The third case was a female patient but there is no information on what she was being treated for with the folic acid therapy. However, after 15 days of folic acid therapy (concentration not specified) she presented with a cutaneous reaction which resolved after 7 days from therapy interruption. There was no history of previous allergic reactions. it was suspected that the reaction was a fixed drug eruption, based on the clinical manifestation and a positive oral provocation test with folic acid (Nucera et al. 2018).
Two more cases were reported in 2020. The first of these cases was a 34-year-old woman who suffered an anaphylactic reaction 5 minutes after ingesting a 400 µg folic acid tablet. The second case was a 30-year-old woman who experienced an anaphylactic reaction with urticaria, dyspnoea, tachycardia, hypotension and lipothymia an hour after taking a 5 mg tablet of folic acid. She was treated in hospital with adrenaline therapy. One month later she consumed a cereal that had been fortified with folic acid and 2 hours later developed an extensive erythematous rash. A skin prick test gave a positive response with folic acid (Gaeta et al. 2020).
FSA contacted two leading UK allergy specialists to seek expert opinion on the prevalence and severity of hypersensitivity to folic acid. They reported that they had not seen any clinical evidence of food allergy or even sensitisation to folic acid in the UK during their careers spanning over 20 years.
The Anaphylaxis Campaign is the only UK wide charity operating solely for people at risk from severe allergic reactions and anaphylaxis. They have a database of people who have contacted them for advice, which includes information on the foods to which they are allergic. There have been no reports of adverse reactions to folic acid.
The Canada Vigilance database and the FDA website were searched for evidence of hypersensitivity reactions linked to folic acid fortification programmes used in Canada and USA, respectively, but no relevant information was found.
The Medicines and Healthcare products Regulatory Agency (MHRA) Yellow Card Interactive Drug Analysis Profile (iDAP) was searched for reports of immune reactions to folic acid between 2000 and 2023, which resulted in a total of 20 reports, with 9 under the category of “Allergic conditions - Hypersensitivity”, 3 under the category of ‘Allergies to foods, food additives, drugs and other chemicals’ and 8 under the category of ‘Anaphylaxis’ (Medicines and healthcare products Regulatory Agency, 2023). The data collected is the result of self-reporting, and the MHRA emphasise that the reported adverse reactions have not been proven to be related to the substance in question and therefore should be interpreted with caution (Medicines and healthcare products Regulatory Agency, 2023).
6. Exposure Assessment
For deterministic allergen risk assessments, the 75th percentile (P75) acute amount of food consumed during a single eating occasion is considered the optimal point estimate to be used, as it meets a safety objective and is adequately conservative within a public health context (Blom et al. 2019). In addition, for this risk assessment, 97.5th percentile (P97.5) acute consumption amounts were also used to represent a worst-case scenario.
The estimated acute consumption (P75 and P97.5) amounts for non-wholemeal wheat flour as an ingredient in different food products such as bread, cakes, pies and pizza (Table 1) were determined using data from the Diet and Nutrition Survey of Infants and Young Children (DNSIYC) and the National Diet and Nutrition Survey (NDNS) (Bates et al. 2014, 2016, 2020; Roberts et al. 2018).
The amount of folic acid consumed if non-wholemeal wheat flour is fortified with folic acid at 250 µg/100g of flour was then estimated and shown in Table 1.
Table 1. Estimated acute 75th percentile and 97.5th percentile consumption of non-wholemeal wheat flour in g/person (with recipes which include a range of food, including breads, cake, pies and pizza that contain flour) and estimated acute consumption of folic acid from non-wholemeal wheat flour fortified at 250 µg/100g flour.
|
Age group |
Number of consumers in data set |
75th percentile* consumption of non-wholemeal wheat flour (g/person) |
Acute folic acid consumption using 75th percentile (µg/person) |
97.5th percentile* consumption of non-wholemeal wheat flour (g/person) |
Acute folic acid consumption using 97.5th percentile (µg/person) |
|
4 - 18 months |
2462 |
39 |
98 |
80 |
200 |
|
Toddlers (1-3 years) |
1151 |
76 |
190 |
110 |
275 |
|
4 - 10 years |
2531 |
110 |
275 |
180 |
450 |
|
11 - 18 years |
2653 |
150 |
375 |
250 |
625 |
|
19 - 64 years |
5028 |
140 |
350 |
250 |
625 |
|
65 + years |
1531 |
100 |
250 |
180 |
450 |
Allergen thresholds
Allergen risk assessments usually involve comparing the amount of allergen consumed during a single eating occasion with population reference doses, for example to determine whether precautionary allergen (or “may contain”) labelling would be appropriate.
Population reference doses are established levels of allergen exposure that would protect the large majority of the allergic population from experiencing an allergic reaction to a food. They are based on data generated from controlled exposure of allergic individuals to low levels of allergen (e.g., where allergenic foods are fed to patients in a controlled hospital setting to test how much allergen they react to). The data are then used to determine a level of exposure at which the majority of the allergic population (e.g., 99% or 95%) would not be expected to react with objective symptoms (although at these levels of exposure, significant subjective symptoms such as gastrointestinal symptoms may result). The aim of reference doses is to protect allergic consumers at the population level; they do not protect every individual on every occasion against every reaction.
A key limitation of the present risk assessment is that reference doses for folic acid have not been established, and so the usual approach for assessing risk could not be followed. Instead, other information from the published literature and other risk assessment bodies was considered.
Published data on amount of folic acid eliciting hypersensitivity reactions
The hazard characterisation section of this risk assessment includes information on the amount of folic acid reported to have elicited hypersensitivity reactions in individual cases in the published literature. The majority of these cases were reported to have consumed supplements containing ≥ 1 mg folic acid. Only two cases were reported to have experienced reactions at levels lower than this, (i.e. one case on two separate occasions at 160 µg and 80 µg and the other case at 400 µg). The 75th percentile and 97.5th percentile amount of folic acid estimated to be consumed on a single occasion if non-wholemeal wheat flour is fortified at 250 µg/100g flour, ranged from 97.5 µg - 375 µg and from 200 µg - 625 µg , respectively, depending on the age and sex of the individual.
Tolerable Upper Limits
Tolerable Upper Limits (TULs) or equivalent for folic acid have been established by a number of risk assessment bodies, including the US Institute of Medicine Food and Nutrition Board (IOM, 1998), the EU Scientific Committee on Food (SCF, 2000) and the UK Expert group on Vitamins and Minerals (EVM, 2003). All of these bodies set a maximum recommended intake of 1 mg/day folic acid based on observations of neurological effects in numerous case series and small studies of folic acid supplementation in patients with pernicious anaemia. Hypersensitivity reactions to folic acid were considered in all of these reports but did not influence the TULs.
In 2018/2019, COT reconsidered the EVM Guidance Level. In its discussion paper on the basis for the Upper Level for Folic Acid (TOX/2018/40), COT considered available information on hypersensitivity from EVM, SCF and IOM, although this was not the main reasoning for Members’ views on the Guidance Level. Instead, when COT reaffirmed the use of 1 mg/day for supplemental folic acid this was on the basis of possible masking of pernicious anaemia in vitamin B12-deficient subjects (COT 2019).
According to the EVM report of Safe Upper Levels for Vitamins and Minerals, a small number of case reports have described hypersensitivity reactions to oral folic acid therapy (generally ≥ 1 mg/day). One short-term, uncontrolled supplementation trial reported adverse symptoms (mental changes (including depression, mild confusion, impaired judgment and difficulty concentrating), sleep disturbances and gastrointestinal symptoms) in healthy volunteers given very high doses of folic acid (15 mg/day) for 1 month to elucidate the effects of folic acid at pharmacological doses on serum vitamin B12 levels, but other studies have not observed similar effects (EVM 2003; Hunter et al. 1970).
The SCF Opinion on the Upper Tolerable Intake Level of Folate indicated that a limited number of case reports have been published on hypersensitivity reactions to oral and parenteral folic acid, but it cannot be excluded that these reactions were due to other components in the formulations. So, hypersensitivity may occur, but is most likely very rare (see Campbell, 1996) (SCF, 2000).
IOM noted that individual cases of hypersensitivity reactions to oral and parenteral folic acid administration had been reported (Gotz and Lauper, 1980; Mathur, 1966; Mitchell et al., 1949; Sesin and Kirschenbaum, 1979; Sparling and Abela, 1985). Such hypersensitivity is rare, but reactions have occurred at supplemental folic acid doses as low as 1 mg/day (Sesin and Kirschenbaum, 1979) (IOM, 1998).
A report commissioned by Food Standards Scotland (FSS) estimated the potential impact of fortification of flour with folic acid in the UK using stochastic modelling. The data in Table 2 shows the modelling information for folic acid at 250 µg/100 g flour and compares this intake to the tolerable upper limits of 1 mg/day for adults set by the EVM and those set for children by the SCF for 1 to 3 year olds (200 µg/day); 4 to 6 years old (300 µg/day); 7 to 10 year olds (400 µg/day); 11 to 14 year olds (600 µg/day); and 15 to 17 year olds (800 µg/day). It should be noted that the tolerable upper limit is based on clinical effects other than hypersensitivity.
Table 2. Effects of fortification of all non-wholemeal wheat flour assuming no capping produced by FSS-funded modelling of fortification with folic acid of 250 µg/100g flour.
|
Age/Gender Group |
Mean folic acid (µg/d) |
Median folic acid (µg/d) |
Tolerable upper limit set by EVM and SCF (µg/d) |
% above UL (folic acid) |
|
1.5 to 3 years males and females |
104 |
98 |
200 |
4.37 |
|
4 to 6 years males and females |
142 |
127 |
300 |
1.38 |
|
7 to 10 years males and females |
166 |
156 |
400 |
1.09 |
|
11 to 13 years males and females |
178 |
171 |
600 |
0.00 |
|
14 to 49 years females |
164 |
127 |
Between 600 and 1000 |
0.47 |
|
14 to 18 years females |
150 |
136 |
Between 600 and 1000 |
0.00 |
|
14 to 18 years males |
198 |
181 |
Between 600 and 1000 |
0.06 |
|
19 to 34 years females |
178 |
129 |
1000 |
0.53 |
|
19 to 34 years males |
204 |
184 |
1000 |
0.00 |
|
35 to 49 years females |
154 |
122 |
1000 |
0.54 |
|
35 to 49 years males |
190 |
163 |
1000 |
0.48 |
|
50 years and over males and females |
182 |
129 |
1000 |
0.69 |
|
50 to 64 years males and females |
173 |
126 |
1000 |
0.71 |
|
65 to 74 years males and females |
183 |
133 |
1000 |
0.47 |
|
75 years and over males and females |
206 |
128 |
1000 |
0.97 |
|
Overall population |
176 |
139 |
NA |
0.63 |
7. Risk Characterisation
The UK prevalence of folic acid hypersensitivity is not known. Cases have been reported in the published literature, although the numbers are small. The UK allergy specialists that were contacted to inform this risk assessment had not seen any clinical evidence of food sensitisation to folic acid in the UK during their careers spanning over 20 years and the Anaphylaxis Campaign has not received any reports of adverse reactions to folic acid. The MHRA Yellow Card Interactive Drug Analysis Profile has a total of 20 self-reported immune reactions to folic acid over the last 23 years. However, it is unknown whether these reactions are related to products fortified with folic acid and they are unproven.
Although hypersensitivity to folic acid may occur it appears to be very rare and linked to higher dose supplements rather than the fortification of food. Only one potential case involving fortification, in which the reaction was mild, could be identified in the literature. It is also important to reiterate that the risk being considered is only for a transitional period of three months, where products will not be labelled as containing folic acid.
Allergen reference doses for folic acid have not been established (e.g., by challenge testing patients in a controlled hospital setting to test how much folic acid they react to), likely because this is not significant at a population level. Consequently, the usual approach for assessing allergen risk could not be followed. However, the 75th percentile and 97.5th percentile/maximum amount of folic acid estimated to be consumed by UK consumers if non-wholemeal wheat flour is fortified with folic acid at 250 µg per 100 g was found to be lower than the amount of folic acid reported to have caused the majority of the adverse reactions in the published literature, with the exception of two cases.
This suggests that while it may be possible for the proposed amount of folic acid in fortified non-wholemeal wheat flour to trigger hypersensitivity reactions, this is only likely to occur very rarely in highly sensitive individuals and would not be significant on a population basis.
In terms of severity of illness, the symptoms of reported allergic reactions to folic acid have ranged from mild to severe (including anaphylaxis) although no deaths have been reported in the published literature. It should be noted that these reactions were linked to the consumption of supplements rather than fortified foods and the majority were triggered by larger amounts of folic acid than has been estimated would be consumed by UK consumers on a single occasion if non-wholemeal wheat flour is fortified with folic acid at 250 µg per 100 g.
In this risk assessment, we used the qualitative scales for the frequency of occurrence and severity of foodborne risks and level of associated uncertainty that is described in the multidimensional risk assessment framework outlined by the Advisory Committee on the Microbiological Safety of Food (ACMSF, 2020), as described in Annex I. The key sources of uncertainty are listed in the next section.
Overall if non-wholemeal flour is fortified with folic acid at 250 µg per 100 g without its presence being labelled on the packaging of the final food or, in the case of food sold loose, not conveyed by other means during a 3-month derogation period then we estimate the risk of hypersensitivity to folic acid in UK consumers to be as follows:
- The frequency of adverse reactions to folic acid in food to be very low (i.e., very rare but cannot be excluded).
- The severity of illness in relation to adverse reactions to folic acid in food to be medium (i.e., moderate illness: not usually life-threatening, sequelae rare, moderate duration).
- The level of uncertainty to be medium (i.e., there are some but no complete data available; evidence is provided in small number of references;).
8. Key sources of uncertainty
The key sources of uncertainty in this risk assessment are:
- The amount of folic acid that needs to be consumed in food on a single occasion to elicit an adverse reaction, given that thresholds have not been established (although there are some data in the published literature on the amount that led to reactions in individual cases). If the amount of folic acid that needs to be consumed in food by sensitive individuals in order to elicit an adverse reaction is lower than anticipated in this risk assessment, then the risk will be higher.
- The amount of folic acid that will be consumed on a single occasion if non-wholemeal wheat flour is fortified with folic acid at 250 µg per 100 g. This risk assessment is based on the 75th percentile and 97.5th percentile data obtained from DNSIYC and NDNS for non-wholemeal wheat flour (with recipes). It is possible that a higher (or lower) amount of fortified flour could be present in the final products and be consumed by an individual leading to a higher (or lower) exposure dose. If the amount of folic acid consumed by sensitive individuals is higher (or lower) than estimated in this risk assessment, then the risk will be greater (or less).
- Folic acid hypersensitivity in the UK population is likely to be very rare but the true prevalence is not known. There is uncertainty regarding whether the limited confirmed clinical data on allergic reactions to folic acid could be due to under-reporting. If the UK prevalence is higher than estimated in this risk assessment, then the risk will be higher.
- A number of case reports used skin prick and/or intradermal testing as confirmation of sensitisation to folic acid, however there are no validated tests available on the market.
- In cases where an immune reaction has been reported as an adverse effect of folic acid, it is often unclear from the patient’s history which foods, supplements or pharmaceuticals that have been recently consumed could have triggered the reaction. By relying on patient self-reporting there is therefore a chance of recall inaccuracies. It is therefore impossible to confirm the cause of those self-reported hypersensitivity reactions leading to the possibility of overestimation of the risk of hypersensitivity to folic acid.
- There is very little information in the literature on possible mechanisms of hypersensitivity to synthetic folic acid. Therefore, it is unclear why synthetic folic acid as opposed to natural folates could trigger a reaction in sensitised individuals.
9. References
Advisory Committee on the Microbiological Safety of Food (ACMSF). Fixed term group on multidimensional representation of risk. (2020):
acmsf_risk_representation_report_1.pdf (food.gov.uk)
Andlid, T., D'Aimmo, M., Jastrebova, J. (2017). Folate and Bifidobacteria. In book: The Bifidobacteria and Related Organisms. DOI: 10.1016/B978-0-12-805060-6.00011-9.
Apraxine, M., Van den Eynde, M., De Cuyper, A., Pirson, F. (2022). Hypersensitivity reactions to folinic acid: mechanisms involved based on two case reports and a literature review. Allergym Asthma and Clinical Immunology, 18:107: Hypersensitivity reactions to folinic acid: mechanisms involved based on two case reports and a literature review | Allergy, Asthma & Clinical Immunology | Full Text (biomedcentral.com)
Bates B, Lennox A, Prentice A, Bates C, Page P, Nicholson S, Swan G (2014). National Diet and Nutrition Survey (NDNS): results from Years 1 to 4 (combined) of the rolling programme for 2008 and 2009 to 2011 and 2012: NDNS: results from Years 1 to 4 (combined) - GOV.UK (www.gov.uk)
Bates B, Cox L, Nicholson S, Page P, Prentice A, Steer T, Swan G (2016). National Diet and Nutrition Survey Results from Years 5 and 6 (combined) of the Rolling Programme (2012/2013 – 2013/2014): NDNS: results from Years 5 and 6 (combined) - GOV.UK (www.gov.uk)
Bates, B., Collins, D., Jones, K., Page, P., Roberts, C., Steer, T., Swan, G. (2020). National Diet and Nutrition Survey Results from years 9, 10 and 11 (combined) of the Rolling Programme (2016/2017 to 2018/2019): NDNS: results from years 9 to 11 (2016 to 2017 and 2018 to 2019) - GOV.UK (www.gov.uk)
Blom, W.M., Remington, B.C., Baumert. J.L., Bucchini, L., Crépet, A., Crevel, R.W.R., Madsen, C.B., Taylor, S.L., Houben, G.F., Kruizinga, A.G. (2019). Sensitivity analysis to derive a food consumption point estimate for deterministic food allergy risk assessment. Food and Chemical Toxicology, 125: 413-421: Sensitivity analysis to derive a food consumption point estimate for deterministic food allergy risk assessment - ScienceDirect
Campbell, N.R.C. (1996). How safe are folic acid supplements? Arch Intern Med 156: 1638-1644: How Safe Are Folic Acid Supplements? | JAMA Internal Medicine | JAMA Network
Centers for Disease Control and Prevention (CDC) (2010). CDC Grand Rounds: Additional Opportunities to Prevent Neural Tube Defects with Folic Acid Fortification. MMWR, 59 (31); 980-984: CDC Grand Rounds: Additional Opportunities to Prevent Neural Tube Defects with Folic Acid Fortification
Centers for Disease Control and Prevention (2022). General information about ntds, folic acid, and folate: General Information About NTDs, Folic Acid, and Folate | CDC (Accessed: 12 September 2023).
Chanarin, I., Fenton, J.C.B., Mollin, D.L. (1957). Sensitivity to folic acid. Brit. Med. J., 1, 1162-1163.
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) (2019). Folic acid – statement on the tolerable upper level (TUL): Folic Acid Statement Final (food.gov.uk)
Crécy-Lagard, V., El Yacoubi. B., de la Garza, D., Noiriel, A., Hanson, A. (2007). Comparative genomics of bacterial and plant folate synthesis and salvage: Predictions and validations. BMC genomics. 8. 245. DOI: 10.1186/1471-2164-8-245. Comparative genomics of bacterial and plant folate synthesis and salvage: predictions and validations | BMC Genomics | Full Text (biomedcentral.com)
Department of Health (2000). Folic acid and the prevention of disease. Report of the Committee on Medical Aspects of food and Nutrition Policy, TSO, London: Folic acid and the prevention of disease. Report of the Committee on Medical Aspects of Food and Nutrition Policy - PubMed (nih.gov)
Dykewicz, M.S., Orfan, N.A., Sun, W. (2000). In vitro demonstration of IgE antibody to folate-albumin in anaphylaxis from folic acid. Journal of Allergy and Clinical Immunology: Volume 106, Issue 2, 386-389: In vitro demonstration of IgE antibody to folate-albumin in anaphylaxis from folic acid - ScienceDirect
Ebara, S. (2017). Nutritional role of folate. Congenital Anomalies, 57: 138–141: doi:10.1111/cga.12233.
EVM (2002). Expert Group on Vitamins and Minerals – Review of folic acid: folicacidrevAug2002.doc (nationalarchives.gov.uk)
EVM (2003). Expert group on Vitamins and Minerals report on Safe Upper Levels for Vitamins and Minerals: Safe Upper Levels for Vitamins
Food Standards Agency (2016). Chief Scientific Adviser’s Science Report Issue five: Food allergy and intolerance: fifth-csa-report-allergy (1).pdf (food.gov.uk)
Gaeta, F., Romano, A., Guéant-Rodriguez, R-M., Guéant, J-L. (2020). IgE-mediated anaphylactic reaction against free synthetic folic acid and methyl folate. The Journal of Allergy and Clinical Immunology: In Practice. Volume 8, Issue 2: 809-811: IgE-mediated anaphylactic reaction against free synthetic folic acid and methyl folate (sciencedirectassets.com).
Gotz, V.P and Lauper, R.D. (1980). Folic acid hypersensitivity or tartrazine allergy? Am J Hosp Pharm: 37(11); 1470-1474.
Hunter, R., Barnes, J., Oakeley, H.F., Matthews, D.M. (1970). Toxicity of folic acid given in pharmacological doses to healthy volunteers. Lancet i: 61-63: Toxicity of folic acid given in pharmacological doses to healthy volunteers - sciencedirect
Institute of Medicine (IOM). (1998). A report of the standing committee on the scientific evaluation of Dietary Reference Intakes and its Panels on Folate, Other B vitamins, and choline and subcommittees on Upper Reference Levels of Nutrients. National Academy Press, Washington D.C.
Mathur, B.P. (1966). Sensitivity of folic acid: a case report. Indian J Med. Sci, 20, 133-134.
Medicines and healthcare products Regulatory Agency (2023). Yellow Card Interactive Drug Analysis Profile: Interactive Drug Analysis Profile - Folic Acid
Milman N. Intestinal absorption of folic acid - new physiologic & molecular aspects. Indian J Med Res. 2012 Nov;136(5):725-8. PMID: 23287118; PMCID: PMC3573592.
Mitchell, D.C., Vilter, R.W., Vilter, C.F. (1949). Hypersensitivity to folic acid. Ann. Intern. Med, 31, 1102-1105.
National Institute of Health (NIH) (2022). Folate Fact Sheet for Health Professionals: Folate - Health Professional Fact Sheet (nih.gov)
NHS (2022): How and when to take folic acid - NHS (www.nhs.uk)
Nucera, E., Aruanno, A., Mezzacappa, S., Pascolini, L., Buonomo, A., Schiavino, D. (2018). Hypersensitivity reactions to folic acid: Three case reports and a review of the literature. International Journal of Immunopathology and Pharmacology. Volume 32: Hypersensitivity reactions to folic acid: Three case reports and a review of the literature (sagepub.com)
Roberts, C.; Steer, T.; Maplethorpe, N.; Cox, L.; Meadows, S.; Nicholson, S.; Page, P.; Swan, G. (2018). NDNS: results from years 7 and 8 (combined). Results of the National Diet and Nutrition Survey (NDNS) rolling programme for 2014 to 2015 and 2015 to 2016: NDNS: results from years 7 and 8 (combined) - GOV.UK (www.gov.uk)
Scientific Committee on Food (SCF) (2000). Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Folate. SCF/CS/NUT/UPPLEV/18 Final. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Folate
Schrijvers, R., Chiriac, A.M., Demoly, P. (2019). Allergy workup for suspected folic acid hypersensitivity. Journal of Investigational Allergology and Clinical Immunology, 25(3): 233-236: Journal of Investigational Allergology and Clinical Immunology 7-16.pdf (jiaci.org)
Sesin, G.P., Kirschenbaum, H. (1979). Folic acid hypersensitivity and fever: a case report. Am. J. Hosp. Pharm, 36, 1565-1567.
Smith, J., Empson, M., Wall, C. (2007). Recurrent anaphylaxis to synthetic folic acid. The Lancet, Vol 370, 652: DFID's health strategy (thelancet.com).
Sparling, R., Abela, M., (1985). Hypersensitivity to folic acid therapy. Clin. Lab. Haematol., 7, 184-185.
Tanno, L., Calderon, M., Demoly, P. (2016). Dissemination of definitions and concepts of allergic and hypersensitivity conditions. The World Allergy Organization Journal, 9:24. https://doi.org/10.1186/s40413-016-0115-2.
Wald, N.J. (2022). Folic acid and neural tube defects: Discovery, debate and the need for policy change. Journal of Medical Screening, 29 (3, 138-146: Folic acid and neural tube defects: Discovery, debate and the need for policy change (sagepub.com).
Woodliff, H.J., Davis, R.E. (1966). Allergy to folic acid. Med, J. Aust., 1, 351-352.
Annex I - Interpretation of probability categories used in this risk assessment
(Tables from ACMSF (ACM/1065) adapted from EFSA 2016 modified from OIE 2004).
|
Frequency category |
Interpretation |
|
Negligible |
So rare that it does not merit to be considered. |
|
Very Low |
Very rare but cannot be excluded. |
|
Low |
Rare but does occur. |
|
Medium |
Occurs regularly. |
|
High |
Occurs very often. |
|
Very High |
Events occur almost certainly. |
|
Severity category |
Interpretation |
|
Negligible |
No effects, or so mild they do not merit to be considered. |
|
Low |
Mild illness: not usually life-threatening, usually no sequelae, normally of short duration, symptoms are self-limiting (e.g. transient diarrhoea). |
|
Medium |
Moderate illness: incapacitating but not usually life- threatening, sequelae rare, moderate duration (e.g. diarrhoea requiring hospitalisation). |
|
High |
Severe illness: causing life-threatening or substantial sequelae or illness of long duration (e.g. chronic hepatitis). |
|
Uncertainty category |
Interpretation |
|
Low |
There are solid and complete data available; strong evidence is provided in multiple references; authors report similar conclusions. |
|
Medium |
There are some but no complete data available; evidence is provided in small number of references; authors report conclusions that vary from one another. |
|
High |
There are scarce or no data; evidence is not provided in references but rather in unpublished reports or based on observations, or personal communication; authors report conclusions that vary considerably between them. |